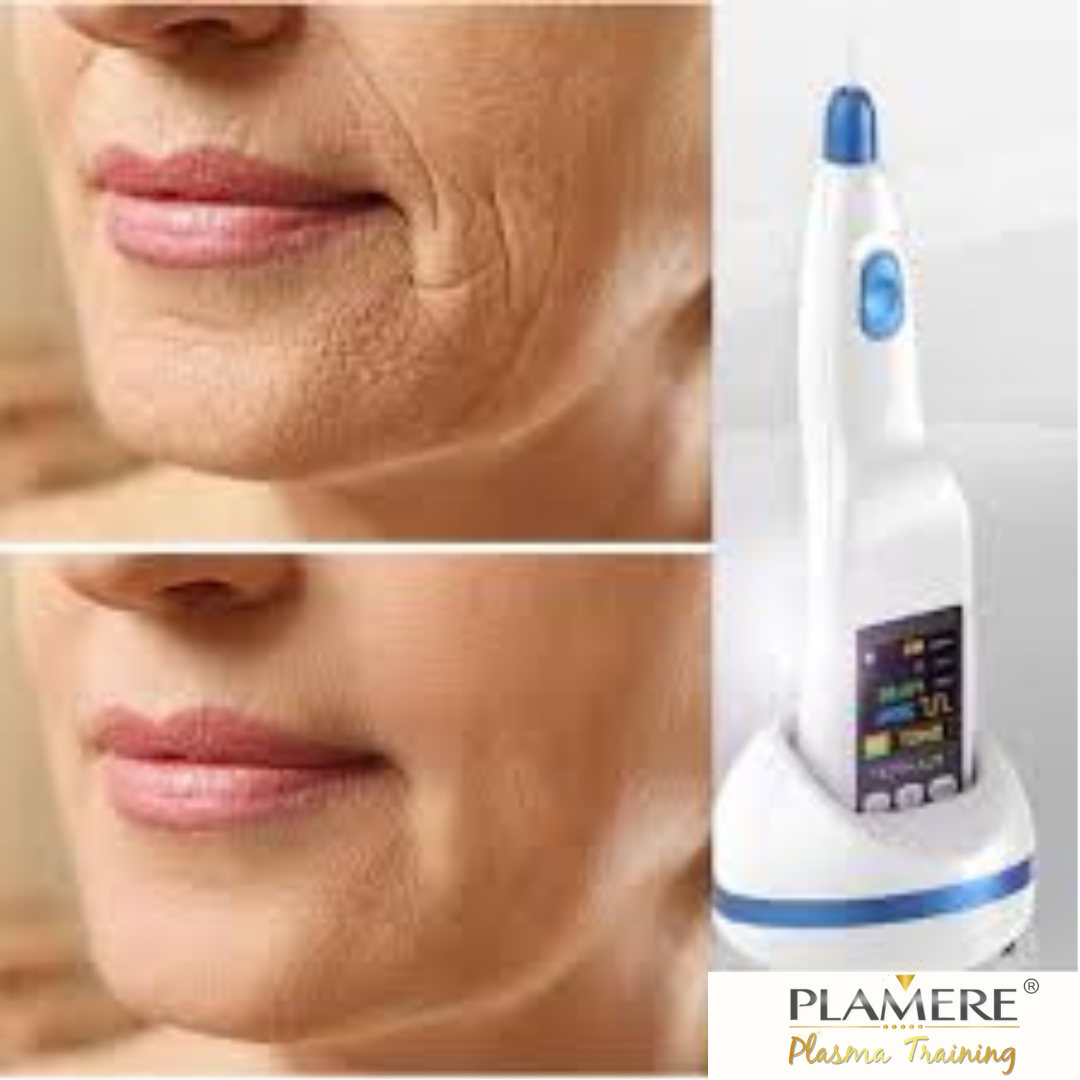
The Plaxpot Plasma Fibroblast Pen: Revolutionizing Skin Rejuvenation with FDA 510(k) Clearance. As distributors of cutting-edge medical devices, we are thrilled to share the exciting news with the world. The Plaxpot Plasma Fibroblast Pen, a groundbreaking technology for non-surgical skin rejuvenation, has recently received FDA 510(k) clearance. This milestone not only underscores its safety and effectiveness but also opens up incredible opportunities for medspa owners looking to offer the latest in skin care treatments.
Let's delve into the world of plasma fibroblast technology, the significance of FDA clearance, and the advantages of the Plaxpot for medspa professionals. Plasma fibroblast is a revolutionary non-invasive cosmetic procedure that utilizes controlled energy to stimulate collagen production and tighten the skin. It is particularly effective for treating a range of skin concerns, including wrinkles, fine lines, sagging skin, acne scars, and stretch marks.
It's crucial for medspa owners to invest in proper training for their staff. We are proud to offer plasma fibroblast training for your service providers. The demand for certified plasma pen technicians is on the rise, and medspa professionals must be equipped with the knowledge and skills to provide safe and effective treatments. Quality plasma pen training programs are essential to ensure that practitioners understand the intricacies of the procedure, including device handling, treatment techniques, and aftercare recommendations. At Plamere Plasma Training we have worked with medspa staff around the world to train their team since the beginning of the launch of plasma pen esthetics.
Our training not only enhances the credibility of your medspa but also ensures that your clients receive the best possible results. The Plaxpot purchase includes our comprehensive training to support medspa professionals in mastering the art of plasma fibroblast treatments. The Significance of FDA 510(k) Clearance The recent FDA 510(k) clearance of the Plaxpot is a game-changer for medspa owners in the USA. This clearance signifies that the Plaxpot meets the FDA's rigorous safety and efficacy standards, making it a reliable choice for skin rejuvenation procedures.
Here are the key benefits of using an FDA-cleared device like the Plaxpot: 1. Safety: FDA clearance ensures that the Plaxpot has undergone rigorous testing and meets stringent safety standards. This provides peace of mind to both medspa professionals and their clients, knowing that the device is safe to use. 2. Efficacy: An FDA-cleared device demonstrates its effectiveness in achieving its intended purpose. Clients can trust that they are investing in a technology that delivers visible results, which is essential for client satisfaction and loyalty. 3. Compliance: Using an FDA-cleared device helps medspa owners maintain compliance with federal regulations. This eliminates potential legal and regulatory challenges, safeguarding your business's reputation and longevity.
Advantages of the Plaxpot Plasma Fibroblast Pen Now that we understand the significance of the FDA clearance, let's explore the unique advantages of the Plaxpot Plasma Fibroblast Pen for medspa professionals:
1. Precision and Control: The Plaxpot offers unparalleled precision and control during treatments. This pen-style device allows practitioners to target specific areas with accuracy, resulting in natural-looking and consistent outcomes.
2. Versatility: The Plaxpot is versatile and can address a wide range of skin concerns, from wrinkles and fine lines to scarring and skin tightening. Medspa owners can offer a variety of treatments with a single device, enhancing their service offerings.
3. Minimal Downtime: Unlike invasive surgical procedures, plasma fibroblast treatments with the Plaxpot require minimal downtime. Clients can return to their daily activities shortly after their session, making it a convenient option.
4. Long-Lasting Results: Plasma fibroblast treatments with the Plaxpot stimulate collagen production, which continues to improve skin quality over time. Clients can enjoy long-lasting results, further increasing their satisfaction.
5. Non-Surgical: The Plaxpot offers a non-surgical alternative to traditional facelifts and invasive procedures. This appeals to clients who seek effective treatments without the risks and recovery time associated with surgery.
6. Comfortable Experience: Plasma fibroblast treatments with the Plaxpot are relatively painless, thanks to the device's precise application and its ability to minimize discomfort. This ensures a comfortable experience for clients.
7. Minimal Side Effects: The Plaxpot produces minimal side effects, such as redness and swelling, which usually subside within a few days. This is another advantage that keeps clients satisfied and returning for more treatments.
8. Marketing Opportunity: Being able to offer an FDA-cleared treatment like the Plaxpot is a significant marketing advantage. It sets your medspa apart from the competition and attracts clients looking for reputable and trustworthy services.
The Plaxpot Plasma Fibroblast Pen, now with FDA 510(k) clearance, is a game-changer for medspa owners in the USA. This advanced technology offers a wide range of benefits, from precise and versatile treatments to minimal downtime and long-lasting results. As a Plaxpot distributor, we encourage medspa professionals to consider incorporating the Plaxpot into their treatment offerings and invest in proper plasma pen training to ensure their team is well-equipped to provide top-notch service. With this FDA clearance, the Plaxpot is poised to lead the way in non-surgical skin rejuvenation, and medspa owners can position themselves at the forefront of this exciting industry. As the demand for safe and effective cosmetic treatments continues to grow, the Plaxpot is the ideal choice for those looking to meet the needs of their discerning clientele and stay ahead in the ever-evolving world of aesthetics.